But Not All Masks Are Created Equal
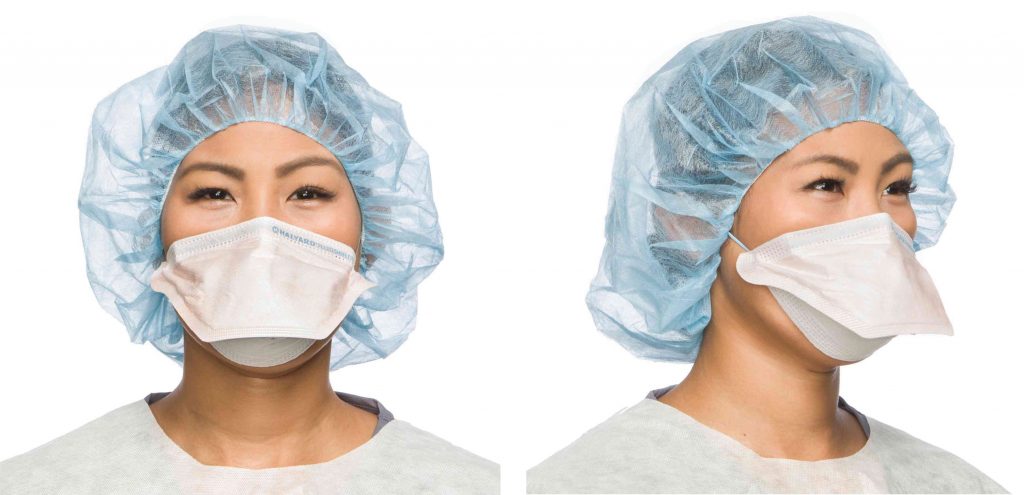
Front and side view of Halyard’s FLUIDSHIELD* Surgical N95 Respirator Mask. It Is considered respiratory protection as it filters out at least 95% of airborne particles including large and small particles.
COVID-19 vaccines are here, but PPE remains essential. The pandemic has presented a myriad of challenges in the healthcare sector, but one of the biggest has been acquiring personal protective equipment (PPE) to help keep clinicians safe. As organizations have turned to vendors at home and abroad to source products, many have discovered that not all masks are created equal.
Medical Masks, N95 Respirators & Medical-Use Approval
Not all medical masks are N95 respirators and not all N95 respirators are approved for use in a medical setting.
According to Laurent Dreyfuss, DO, an emergency physician and Director of Global Medical and Clinical Affairs at O&M Halyard, medical face masks can be grouped into three categories: Procedure Masks, Surgical Masks, and N95 Respirators. While all three categories are sometimes referred to as “medical masks,” there are important differences between them.
Procedure masks are held to the face with stretch loops behind the ears. Surgical masks, intended for use during surgery, have two sets of ties to ensure a more secure fit. Respirators typically have over-the-head elastic bands to hold them firmly in place.
Halyard’s FLUIDSHIELD Surgical N95 Respirator Mask
Further, respirators typically have higher levels of filtration performance than procedure and surgical masks. And respirators are designed to provide a better seal which provides better protection to the wearer than procedure masks. Healthcare organizations must determine what type or types of masks are best suited for their employees. When selecting masks, healthcare providers should consider several questions.
The Most Protective Type of Mask – NIOSH-Certified
What is the hazard and how infectious is it? Is the hazard transmitted by contact, droplets, or airborne? And if it’s airborne, what is the particle size of the hazard?
With novel pathogens like COVID-19, the answers to these questions may not be clearly known. As a result, the CDC recommends using the most protective type of mask, the N95 respirator, if there is a risk that the pathogen could be transmitted by respiration. Healthcare organizations must also recognize even within the respirator category, important distinctions exist. The first purchase consideration is whether the National Institute for Occupational Safety and Health (NIOSH), an agency of the CDC, has certified the respirators in question. Only NIOSH-approved respirators can be called N95s. NIOSH-certified N95 respirators meet federal standards for breathability and filtration. They must show the ability to filter out at least 95 percent of very small particles (defined as 0.3 microns) from the air at high flow rates. In addition, the system quality used in the manufacturing of N95s must be approved and routinely audited by NIOSH.
“Not all N95 respirators are NIOSH certified. As healthcare providers search for PPE, they may see references to KN95 respirators. These masks are made and certified in China [by the National Medical Products Administration]. They have not been tested and certified by NIOSH,” notes Dr. Dreyfuss.
FDA Clearance
Another key purchasing criterion for N95 respirators is whether or not they have also been cleared by the FDA for use as medical face masks. The FDA requires respirators for medical use to also meet the criteria for flame resistance, biocompatibility, and the ability to resist penetration by splashes from fluids such as blood. The ASTM F2100 standard for medical face masks establishes three levels of splash resistance. Level 1 roughly correlates to low, 80mmHg blood pressure; Level 2 at 120mmHg blood pressure; and Level 3, the highest rating, is 160mmHg blood pressure.
Halyard’s FLUIDSHIELD Surgical N95 Respirator Mask
Just as not all N95 respirators are NIOSH certified, not all N95s are ASTM rated. Many N95 respirators are designed for industrial settings, rather than medical environments.
Surgical Masks vs. N95 Respirators
Surgical Mask |
N95 Respirator |
||||
|---|---|---|---|---|---|
| Approval & Regulations | Cleared by the U.S. Food and Drug Administration (FDA) and regulated under 21 CFR 878.4040. |
Regulated by the FDA, CDC, NIOSH, OSHA. Evaluated, tested, and approved by NIOSH as per the requirements in 42 CFR Part 84. | |||
| Intended Purpose | Fluid resistant. Reduces wearer’s exposure to and provides protection against large droplets, splashes, or sprays of bodily or other hazardous fluids. Protects the patient from the wearer’s respiratory emissions. | Reduces wearer’s exposure to particles including small particle aerosols and large droplets (only non-oil aerosols). Designed to protect both the patient and healthcare professional from the transfer of microorganisms, bodily fluids, and particulate material at an N95 filtration level per 42 CFR 84.181. | |||
| Use Limitations | Disposable. Not intended to be used more than once and should be discarded if damaged, soiled, or if breathing through the mask becomes difficult. | Ideally should be discarded after each patient or customer encounter. It should also be discarded when it becomes damaged or deformed; no longer forms an effective seal to the face; becomes wet or visibly dirty; breathing becomes difficult, or it becomes contaminated with any fluids from patients or customers. | |||
| Filtration | Not considered respiratory protection. Does NOT provide the wearer with a reliable level of protection from inhaling smaller airborne particles. | Is considered respiratory protection. Filters out at least 95% of airborne particles including large and small particles. | |||
| Air Leakage | Leakage occurs around the edge of the mask when the user inhales. | When properly donned and fitted, minimal leakage occurs around the edges of the respirator when the user inhales. | |||
| Fit Test Requirement | Not required | Yes, per OSHA standard 1910.134 App A. | |||
| User Seal Check Requirement | Not required | Yes. Required each time the respirator is put on, known as donning. | |||
| 1. Centers for Disease Control & Prevention, “Understanding the Difference, Surgical Mask, N95 Respirator,”(Retrieved May 12, 2021) 2. USFDA, “N95 Respirators, Surgical Masks, and Face Masks,” (Retrieved May 12, 2021) 3. USFDA, “Memorandum of Understanding Between the Food & Drug Administration et al,” (Retrieved May 12, 2021) |
|||||
ASTM Mask Ratings
These products may have no ASTM rating or they may have a lower performance rating, such as Level 1 or Level 2,” says Dr. Dreyfuss. Once you’ve acquired N95 respirators, fit and employee use cannot be overlooked. Unlike surgical or procedure masks, OSHA mandates N95 respirators must be fit tested for each wearer. Fit testing ensures a good seal to the face so respirated air passes through, not around, the respirator.
If organizations purchase different brands of N95 respirators, employees must be fit tested for each brand. High levels of protection depend on a snug fit that is unique for each individual.
With the COVID-19 vaccine rollout, hopes are high life will eventually return to normal. In the months ahead, however, masks and other PPE will continue to be essential to keep providers safe. Healthcare organizations should consult FDA requirements to ensure they are using properly certified equipment.
Written by Halyard
Permission to publish provided by Halyard.
Published in TIPS – July/August
Category: Merchandise
Back to Articles